8
hours
of pain relief on average following a 30-minute treatment¹
8
of pain relief on average following a 30-minute treatment¹
94 %
after using BioWave for two weeks¹
54 %
after using BioWave for two weeks1
3.47
after using BioWave2
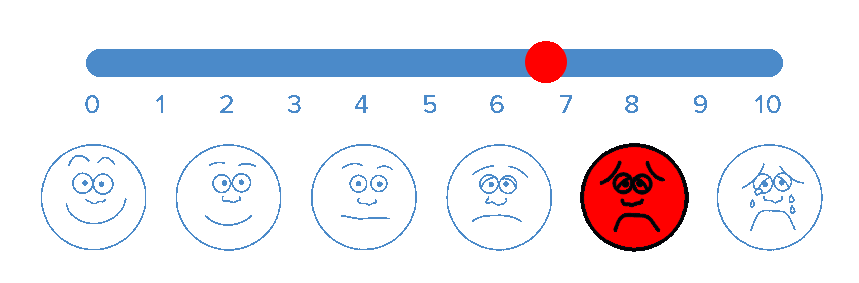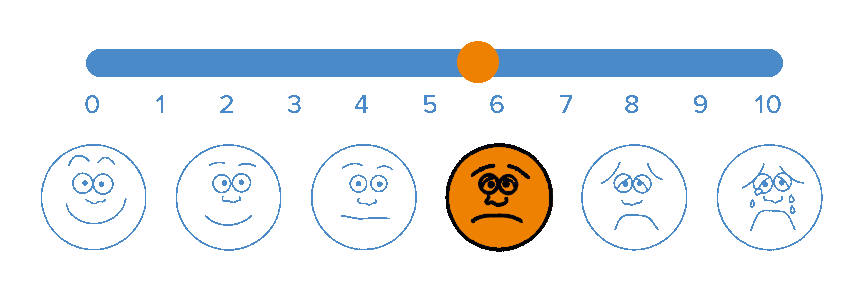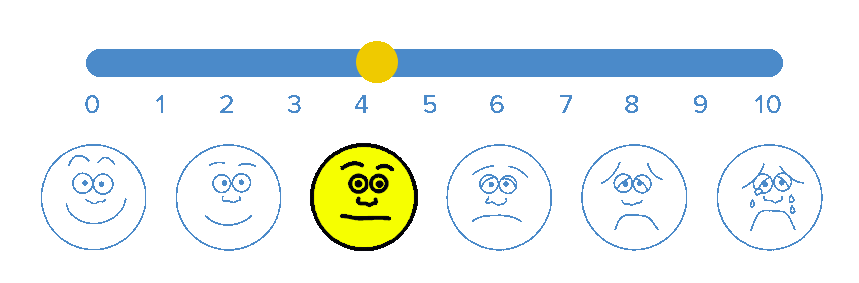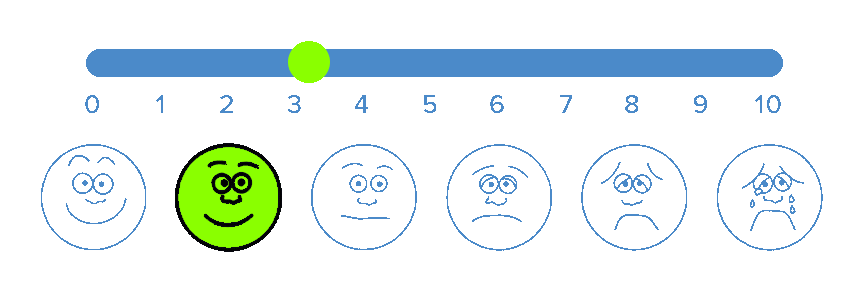
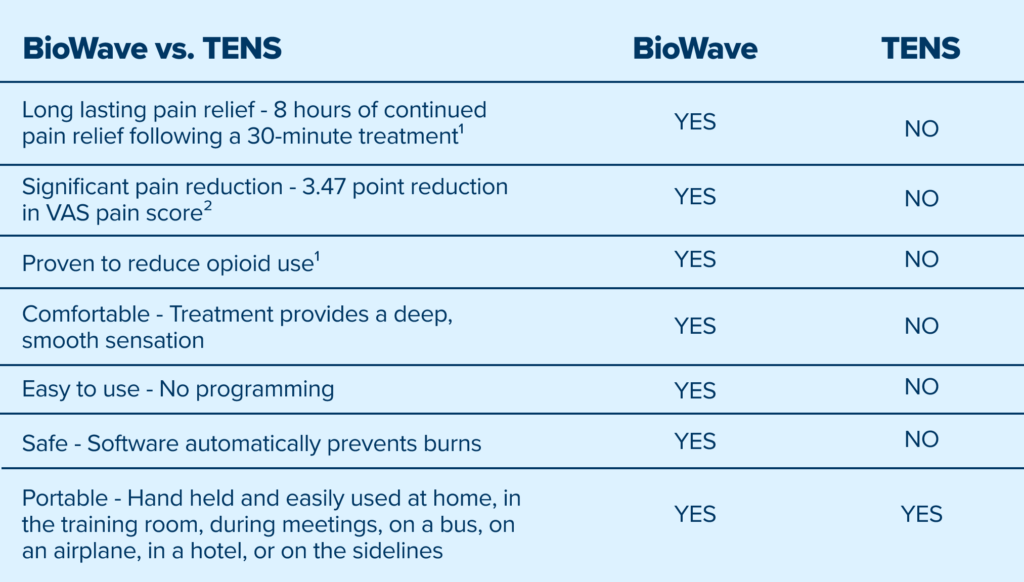
BioWave’s patented high frequency neurostimulation is delivered through skin directly to pain nerves, inhibiting action potential propagation along the nerve, blocking the transmission of pain signals to the brain.
Featuring BioWaveHOME
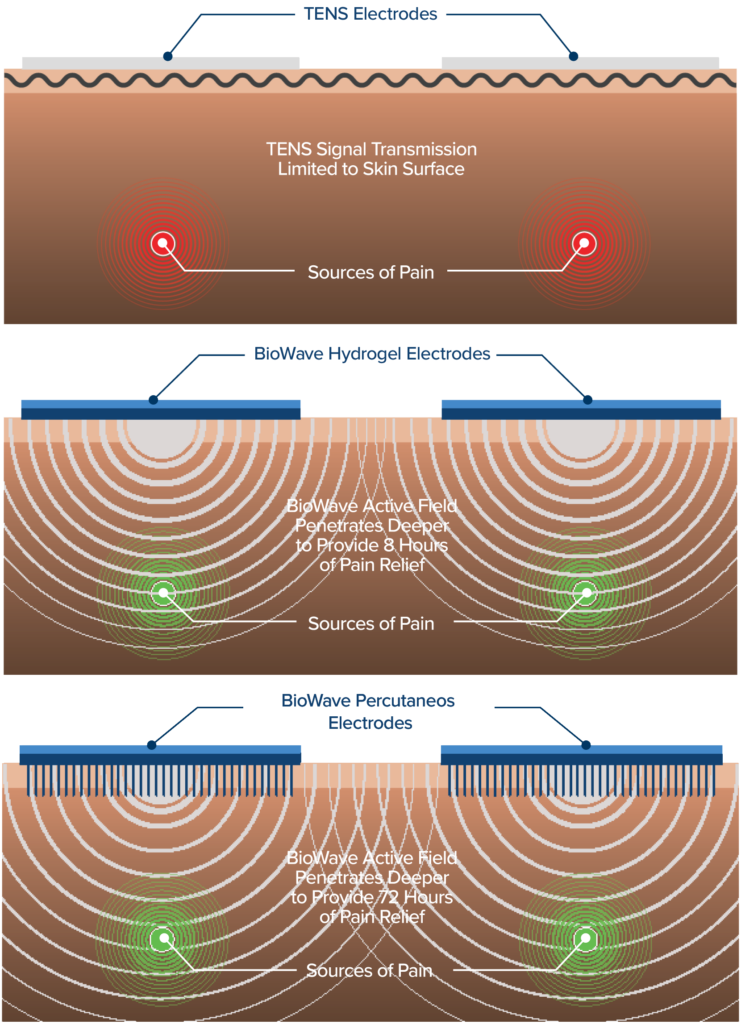
Unlike TENS and other electrical stimulation devices that only mask pain at the skin’s surface, BioWave’s patented signal technology delivers therapeutic stimulation deep into the body, directly to nerves, blocking signals at their source.
This results in a greater magnitude and longer duration of pain relief, often for many hours after a single 30 minute treatment, as well as improved mobility without the need to keep the device on continuously.
 Neck Pain
Neck Pain
How BioWave Reduced Pain and Improved Function and Quality of Life in Chronic Neck and Shoulder Pain In this case study, Charles A. Odonkor, MD, MA, shares how…
 Sports Injury Applications
Sports Injury Applications
How BioWave Became Part of an MLB Pitcher’s 5-Day Recovery Cycle In this case study, Rick Griffin, BS, MS, ATC-L, Seattle Mariners Athletic Trainer Emeritus, shares how BioWave…
 Back Pain
Back Pain
How BioWavePENS Reduced Whiplash-Related Lumbar Pain Without Injections In this case study, Raymond Tatevossian, MD, shares the treatment of a 25-year-old male with lumbar pain following a motor…
Open-label survey of chronic pain patients recruited from Veteran Affairs, orthopedic and pain health systems. This retrospective pilot study is shaped around a noninvasive…
Following two weeks of BioWaveHOME use, four hundred sixty three (463) subjects provided responses via returned surveys. The BioWaveHOME device provided subjects with statistically…